Which Best Describes an Element a Pure Substance
Everything in the universe is made from elements. Answer is a pure substance.
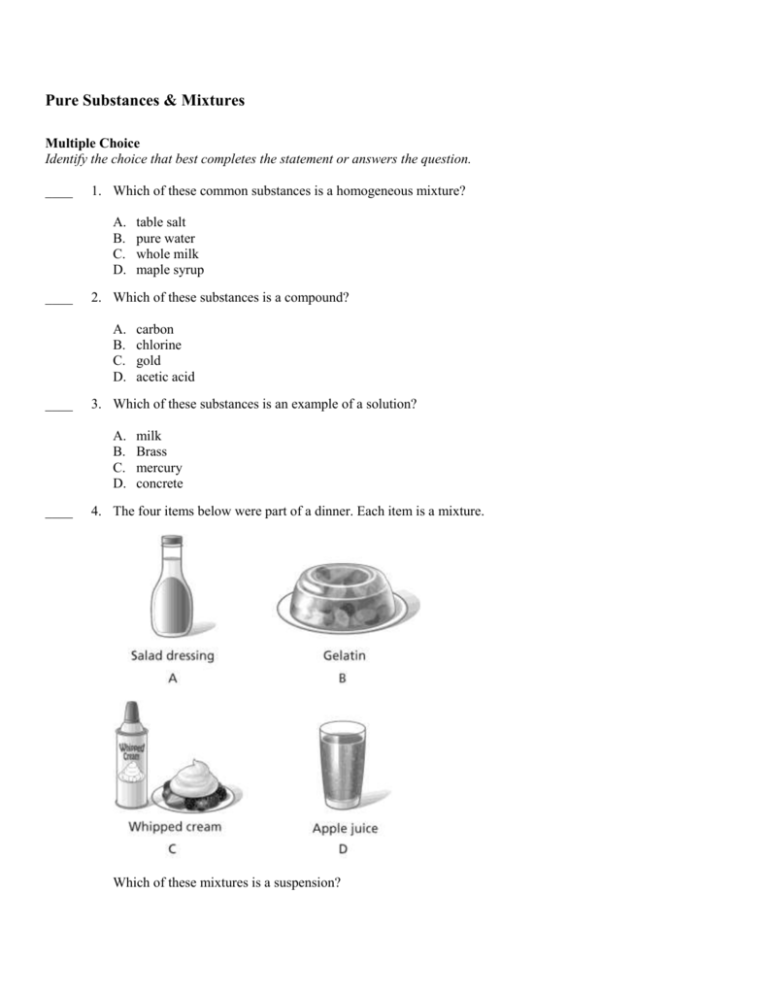
Pure Substances Mixtures Multiple Choice Identify The Choice That
B a pure substance.

. -a pure substance because it is a compound. A mixture of two uncommon substancesc. A type of mixture c.
A pure substance a type of a mixture a pure compound an impure substance. Answer choices elements mixtures compounds Question 11 10 seconds Q. Which best describes a compound such as magnesium oxidea.
-an impure substance because it is a mixture. The chemical formula for emerald is Be3AL2 SiO36. A pure substance because it is an element.
An emerald can be described as. THIS SET IS OFTEN IN FOLDERS WITH. Upload your study docs or become a Course Hero member to access this document.
A pure substance that can be separated into different elements by physical means. It is the purest and most basic form of any substance on Earth. An impure substance that is made up of two elementsb.
Because an element can have more than one type of atoms as in case of isotopes. Elements are chemicals that are pure substances represented by symbols that have at least one capital letter D. A pure substance because it is a compound.
An impure substance because it is chemically combined. Elements are atoms that are made of subatomic. Bromine a liquid at room temperature has a boiling point of 58C and a melting point of -72C.
CHON and P can be taken as examples for elements. A mixture of two common substances that can be separated by chemical means. An emerald can be described as.
-a pure substance because it is an element. A pure substance made up of only one kind of atom. It can be separated through a chemical process D.
Which best describes an element. Elements are mixtures of protons neutrons and electrons in different combination C. A pure substance that is made up of different elements.
An impure substance because it is chemically combined. An element has unique number of protons and electrons. Elements that are chemicals that are formed when temperature change happens or a precipitate forms.
Bromine can be classified as a compound. An impure substance because it is a mixture. It is an element because it is made from a pure substance.
A mixture of two common elements that can be separated by chemical means. Mitgliedd1 and 2 more users found this answer helpful. Which statement best describes an element.
Rock layers were found containing 30 kg. Which of the following changes is a physical change of matter. A pure substance b.
Answer choices element mixture compound Question 10 10 seconds Q. Answer choices elements mixtures compounds Question 12 30 seconds Q. Any combination of two or more atoms of different types.
Any kind of crystal. Which best describes a compound such as sodium chloride. A substance containing only carbon atoms.
It can be broken down into other type of substances C. An emerald can be described as Click card to see definition a pure substance because it is a compound Click again to see term 110 Previous Next Flip Space. Which best describes an element.
An element is. Which of the following describes an element. A pure substance a type of a mixture a pure compound an impure substance A.
An emerald can be described as a pure substance because it is a compound. A pure substance a type of a mixture a pure compound an impure substance. -an impure substance because it is chemically combined.
It is composed of two or type of atoms. Examples are water H 2 O and carbon dioxide CO 2. A pure substance because it is an element.
A pure substance containing only one kind of atom. The chemical formula for emerald is Be3Al2 SiO36. A pure compound d.
Which best describes an element. An impure substance because it is a mixture. A pure substance a type of a mixture a pure compound an impure substance Previous Dé 2 razones por las que a un automovilista menor de 21 años se le puede permitir conducir después de las restricciones de.
Want to read all 2 pages. In other words we can say an element has one type of atoms. Due to the number of neutrons present in the nucleus an element can be either stable or radioactive.
A pure substance because it is a compound. A mixture of two common elementsd. These are found on the Periodic Table.

Mixtures And Pure Substances Heterogeneous Mixture Pure Products Mixtures
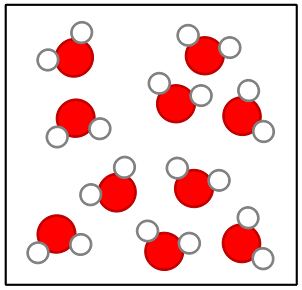
Elements Compounds And Mixtures Science Quiz Quizizz

Saturated Solution Easy Science Easy Science Chemistry Experiments Solutions

Question Video Identifying The Name Of A Substance Consisting Of One Type Of Atom That Cannot Be Broken Down Chemically Into A Simpler Substance Nagwa

Lesson Explainer Mixtures Nagwa

Pure Substances And Mixtures Elements Compounds Classification Of Matter Chemistry Examples Youtube

How Elements Are Formed Teaching Resource Our World Is Made Of Elements And Combinations Of Elements Called Compoun Neon Light Signs Neon Signs Neon Lighting

Elements Compounds And Mixtures Pixel Art Digital Review Distance Learning Science Teaching Resources Compounds And Mixtures Teaching Chemistry

Robot Check Unreal Engine Physics Concepts Physics

Selina Icse Solutions Class 9 Chemistry Elements Compounds Mixtures 3b 3 Https Www Aplustopper Com Selina Compounds And Mixtures Chemistry Chemical Science

What Gave The Name To The Indium Chemical Element Indigo Dye In 1863 The German Chemists Ferdinand Reich And Hieronymous T Chemical What Gives Indigo Colour

Lakhmir Singh Chemistry Class 10 Solutions Carbon And Its Compounds Chemistry Education Chemistry Basics Chemistry Class

Elements Compounds And Mixtures 3 Worksheets Answers Teaching Resources Compounds And Mixtures Chemistry Worksheets Matter Worksheets

Unit 7 Nuclear And Kinetics School Cartoon Teaching Science Electrochemistry

Dr Masaru Emoto S Water Crystals Show Us The Power Of Words Masaru Emoto Powerful Words Hidden Messages In Water

Why Are Elements And Compounds Pure Substances Lisbdnet Com

What Is A Pure Substance Youtube

Comments
Post a Comment